Abstract
Background: Our understanding of the biology of acute myeloid leukemia (AML) has increased dramatically with the use of next-generation sequencing. The identification of recurrently mutated genes in AML has allowed for better risk stratification and provided novel therapeutic targets. The European Leukemia Network (ELN) 2017 prognostic system divides patients into favorable, intermediate, and adverse groups based on genetic abnormalities.[Döhner 2017] Patients with features including MDS related changes, complex karyotype and adverse molecular features including mutations in FLT3 are at high risk (HR AML) for treatment failure and relapse.
Aim: This study was conducted to determine if an online, simulation-based continuing medical education (CME)-certified intervention could improve clinical decision making of hematologists/oncologists (hem/oncs) regarding treatment selection for patients with HR AML.
Description of Intervention: A CME-certified virtual patient simulation (VPS) was made available via a website dedicated to continuous professional development. The VPS consisted of 2 cases of HR AML presented in a platform that allows hem/oncs to assess the patients and make clinical decisions supported by an extensive database of diagnostic and treatment possibilities, matching the scope and depth of actual practice.
*CASE 1* 63-year-old male with AML-MRC, mutations detected: RUNX1, TET2, SRSF2; no mutations detected in NPM1, CEBPA, IDH1, IDH2, KIT, KRAS, NRAS, ASXL1, ASXL2, BCR-ABL1, WT1.
*CASE 2* 57-year-old female with FLT3-ITD mutated AML who is a candidate for intensive induction therapy.
Methods: Clinical decisions were analyzed using a sophisticated decision engine, and tailored clinical guidance (CG) employing up-to-date evidence-base and faculty recommendations was provided after each decision. Decisions were collected post-CG and compared with each user's baseline (pre-CG) decisions using McNemar's test to determine p-values (P < .05 indicates significance). Data were collected between October 8, 2020 and July 22, 2021.
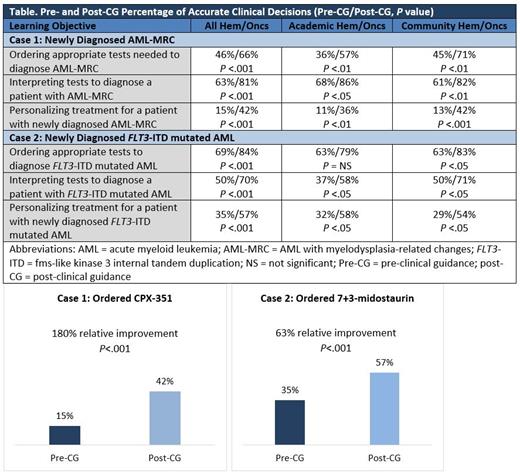
Results: At the time of assessment, 186 hem/oncs who made clinical decisions were included in the analysis (112 case 1, 74 case 2). From pre- to post-CG in the VPS, hem/oncs were significantly more likely to make evidence-based practice decisions across all learning objectives, see the Table.
*CASE 1* For the case of AML-MRC, the VPS led to a higher percentage of community-based hem/oncs ordering necessary diagnostic tests and ordering an appropriate treatment. After clinical guidance, slightly more academic-based hem/oncs identified the correct diagnosis for the patient.
*CASE 2* For the patient with FLT3-ITD mutated AML, the VPS led to a higher percentage of community-based hem/oncs ordering and correctly interpreting diagnostic tests in order to make an accurate diagnosis. After clinical guidance, a higher percentage of academic-based hem/oncs ordered an appropriate treatment for the patient based on the FLT3-ITD mutation and their fitness assessment. Treatment rationales were collected and can be presented.
Conclusions: This study demonstrates that VPS that immerses and engages hem/oncs in an authentic learning experience improved evidence-based clinical decisions related to the management of HR AML. Clinical guidance in the VPS improved hem/oncs clinical decision making for all learning objectives and the improvements were statistically significant. For almost all learning objectives, the activity had a significant and larger impact on improving clinical decision making of community-based hem/oncs compared to hem/oncs from other practice settings. This study indicates that unique educational methodologies and platforms, which are available on-demand, can be effective tools for promoting guideline-based therapy selection and clinical decision making. Additional education is recommended about the role for FLT3 inhibitors and the optimal treatment for AML-MRC.
Acknowledgement: This CME activity was supported by an independent educational grant from Jazz Pharmaceuticals.
References:
1. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. 2017. 129(4):424-447.
2. MedSims Activity: https://www.medscape.org/viewarticle/936156
Stone: AbbVie Inc, Actinium Pharmaceuticals Inc, Aprea Therapeutics, BerGenBio ASA, ElevateBio, Foghorn Therapeutics, GEMoaB, GlaxoSmithKline, Innate Pharma, Syndax Pharmaceuticals Inc, Syros Pharmaceuticals Inc, Takeda Oncology: Other: Advisory Committee; Agios Pharmaceuticals Inc, Novartis;: Research Funding; ACI Clinical, Syntrix Pharmaceuticals, Takeda Oncology: Other: Data Safety & Monitoring. Uy: GlaxoSmithKline: Consultancy; AbbVie: Consultancy; Agios: Consultancy; Macrogenics: Research Funding; Astellas: Honoraria, Speakers Bureau; Novartis: Consultancy; Genentech: Consultancy; Jazz: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal